Norwest is proud to support innovative healthcare companies that deliver better patient outcomes at lower cost. One such company is Vertos Medical and we are delighted to announce Norwest has led a $26 million Series C financing for Vertos.
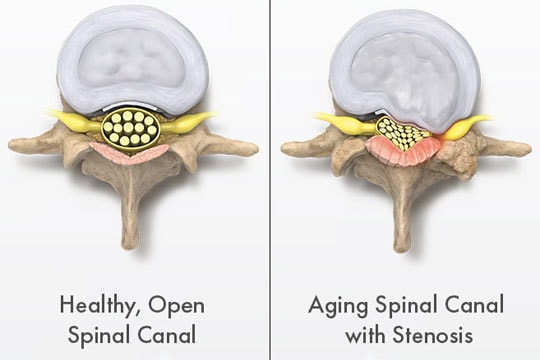
Vertos addresses lumbar spinal stenosis (LSS), a narrowing of the spinal canal that causes pain and loss of strength in the lower back and legs and is a condition that affects nearly 15 million people in the U.S. Vertos treats LSS with a minimally invasive outpatient procedure that has demonstrated higher rates of relief with fewer complications at lower cost than traditional procedural and surgical treatment methods.
LSS Causes Millions to Experience Pain and Limited Mobility
Within the spinal canal is the spinal cord, which is essentially a bundle of nerves that connects the brain with the rest of the body. LSS is a degenerative disease that narrows the spinal canal in the lower part of the back (i.e., the “lumbar” area), which in turn increases the pressure on the spinal cord and/or the nerves leaving the spinal cord.
Patients with LSS experience pain in their lower backs and/or legs and have difficulty walking and standing for extended periods. Because LSS is primarily caused by age-related deterioration of the bones and soft tissue in the lumbar vertebral column, the prevalence of LSS increases with age. For people between the ages of 60 and 70, an estimated 20 to 25 percent suffer from LSS. LSS is the most common reason for spinal surgery in patients over age 65.
Conventional medical management (CMM) for LSS includes anti-inflammatory medications like NSAIDs (e.g., Tylenol, Advil, etc.) to reduce swelling and physical therapy. For patients who don’t achieve relief from CMM, the next step usually is epidural steroid injections (ESIs), where a doctor injects a corticosteroid into the point of spinal narrowing to reduce the inflammation and swelling at the nerve. Unfortunately, steroid injections usually provide only modest and temporary relief, so most patients undergo multiple ESIs in a year with limited to no long-term pain relief.
Historically, LSS patients who have tried ESIs with no success, have only surgery as the remaining treatment option – a laminectomy and spinal decompression with or without a spinal fusion. Spinal decompression surgery for LSS is highly invasive, generally requires an inpatient hospital stay, and is very expensive ($40K+). Efficacy for surgery is quite limited – up to 40 percent of patients suffer failed back surgery syndrome (FBSS). The surgery also has a high rate of re-operations, with as many as 17 percent of patients requiring additional surgery within two years.
A recent study of surgical outcomes in LSS patients found that 32 percent were not satisfied with their outcomes after 5 and 10 years (condition improved only slightly, didn’t improve at all, or worsened).
Clearly, there is a need for an alternative that is more effective, less costly, and easier on patients.
The Vertos Solution Addresses All the Drawbacks of Current Treatment Options
That alternative is already in use: Vertos Medical’s mild® procedure (see video below). It is performed in an outpatient setting and takes about 40 minutes. It requires an incision of only 5mm (the size of an aspirin), through which an interventional pain specialist removes parts of the bone and soft tissue that are causing the narrowing of the spinal canal. Most patients return home the same day and typically resume normal activity within 24 hours with no restrictions.
The main mechanism of action is thinning the ligamentum flavum (LV), a ligament inside the spinal canal that frequently becomes thickened in patients with LSS. While most LSS patients have multiple factors for spinal canal narrowing, the mild procedure accomplishes the primary goal of treating LSS: decompressing the spinal canal and reducing pressure on the spinal cord and nerves.

The mild procedure was initially approved by the FDA in 2010 and in 2017, following multi-center, randomized clinical trials that demonstrated efficacy, the mild procedure was granted a national coverage determination (NCD) by the Centers for Medicare and Medicaid Services (CMS).
For example, a study by Loma Linda VA Healthcare showed that 50 percent of patients were discharged from chronic pain management treatments after the mild procedure. It also noted a 75 percent reduction of interventional pain management procedures. A study by the Cleveland Clinic found that the average cost of the mild procedure was 60 percent less than laminectomy surgery (77 percent less when all related hospital charges were included).
Our interviews with pain management physicians have been very positive, with nearly all of them expecting to increase their case volumes in coming years. Physicians appreciate the simplicity of the procedure and have been impressed with both efficacy and safety results. They also appreciate that the mild procedure doesn’t require an implant or limit future procedures.
Vertos Serves a Large Market with Strong Competitive Advantages
The Norwest team is excited about Vertos for several reasons.
- Outcomes – The mild procedure is uniquely positioned as the only minimally invasive LSS treatment with proven long-term efficacy and a positive safety profile, and it does not require an implant.
- Market size – Nearly 15 million patients in the U.S. experience symptomatic LSS. Within that population, 6.8 million have been clinically diagnosed with LSS and 4.8 million are actively seeking care. Of these, 2.4 million are candidates for the mild procedure. Vertos has the strongest reimbursement coverage with Medicare, which covers about 55 percent of the addressable patient population – creating a $3.2B market opportunity.
- Market adoption and growth – More than 70,000 patients have been treated with the mild procedure in the U.S., and between 2017 and 2022 Vertos experienced a compound annual growth rate in sales of 75 percent.
- Seasoned management – Vertos has a strong, experienced, and cohesive management team. CEO Eric Wichems and CFO/COO Rebecca Colbert have both been with the company for over a decade, spearheading the team through the pivotal clinical trial and deep into commercialization.
We believe Vertos is a sterling example of exactly what the U.S. healthcare system needs: an innovative therapeutic approach that improves patient outcomes, shortens treatment times, lessens complications, and reduces costs.
We are pleased to partner with Vertos management and other investors to drive further growth.